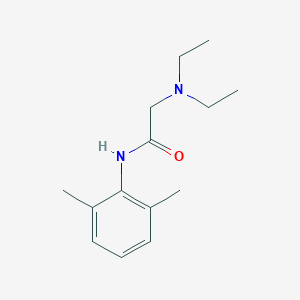
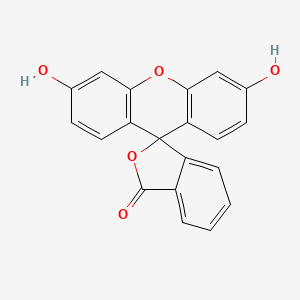
Diagnostic agent for the measurement of intraocular pressure by Goldmann tonometry
Adult: Lidocaine 4% w/v and fluorescein 0.25% w/v eye drop solution
Instil 1 or more drops, as required.
Instil 1 or more drops, as required.




 Sign Out
Sign Out